TSX-049
A differentiated oral Testosterone Replacement Therapy
TSX-049 is a novel, oral formulation of Testosterone (T) containing Testosterone Undecanoate (TU) that is designed to help restore normal Testosterone levels in males with hypogonadism.
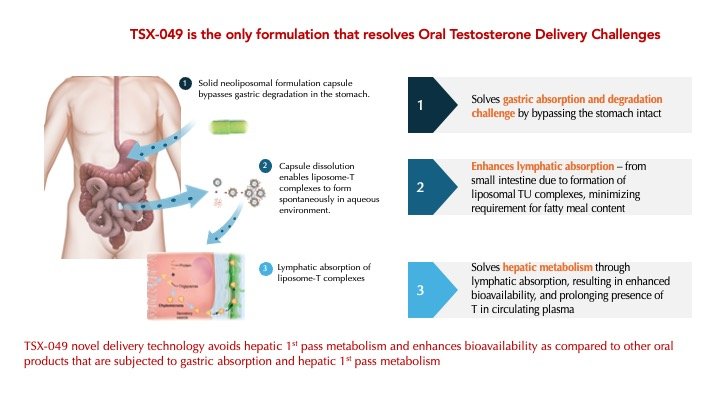
By utilizing TesoRx's proprietary neoliposomal formulation platform to deliver T, TSX-049 is anticipated to have the following advantages:
No supra-physiological peak T levels
Minimal food effect, and no requirement for a high fat meal
Optimized PK profile with potential for once-a-day dosing
Greater safety and convenience compared to existing standard of care treatments
TesoRx completed a successful confirmative study in canines that was designed to predict the pharmacokinetic profile of TSX-049 for the oral treatment of hypogonadism. The results of the study confirmed that the optimized TSX-049 formulation has enhanced bioavailability and a dose response sufficient to overcome negative feedback of gonadal testosterone production. TesoRx will be advancing TSX-049 into Phase 1-2a human clinical trials and anticipates a potential U.S. Food and Drug Administration approval in early 2027.
Significant unmet need
Approximately 39 percent of men over the age of 45 are believed to be hypogonadal and potentially eligible for Testosterone Replacement Therapy (TRT). Current testosterone products come with significant lifestyle restrictions for patients like needing to cover their skin or not have physical contact to reduce the risk of testosterone transference to loved ones and sub optimal performance.
T-Trials funded by the NIH results show TRT benefits men
A comprehensive study, led by the Perelman School of Medicine at the University of Pennsylvania and funded by the National Institutes of Health involved 790 men 65 and older with low testosterone levels for their age.
Men reported moderate increases in their interest in sex, their performance and erections. There was also a smaller degree of improvement in mood, vitality and walking activity.
Primary Outcomes in the Three Main Trials of the Testosterone Trials.
Sexual function
The primary outcome of the Sexual Function Trial (Panel A) was the change from baseline in the score for sexual activity (question 4) on the Psychosexual Daily Questionnaire (PDQ-Q4; range, 0 to 12, with higher scores indicating more activity).
Physical Function
The primary outcome of the Physical Function Trial (Panel B) was the percentage of men who had an increase of at least 50 m in the distance walked during the 6-minute walk test.
Vitality
The primary outcome of the Vitality Trial (Panel C) was the percentage of men who had an increase of at least 4 points in the score on the Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scale (range, 0 to 52, with higher scores indicating less fatigue). P values were calculated with the use of a linear random-effects model for sexual activity and logistic random-effects models for walking ability and vitality. The I bars represent standard deviations.